
University of New South Wales Law Journal


|
Home
| Databases
| WorldLII
| Search
| Feedback
University of New South Wales Law Journal |

|
ANNETTE URQUIJO[*]
Medical science has traditionally been seen as a non-economic endeavour;[1] however the advent of patented methods of medical treatment[2] has proven this view to be outdated. This transition has been the subject of widespread debate as to the ethics of allowing the economic rationale of patent law to enter the humanity driven area of medical science. In particular, the most quoted ethical concern against the patenting of methods of medical treatment is that access to healthcare will be denied or restricted in a deleterious manner.
However, the argument has been rejected as a legal challenge to granting a patent over methods of medical treatment,[3] as there is little scope under the Australian Patents Act 1990 (Cth) for the consideration of ethical issues.[4] The main concern of the Australian Patents System is assessing whether the invention meets the technical requirements for patentability.[5] The Australian Law Reform Commission argues that the social and ethical issues involved in patent law, are left to be addressed by other areas of the law.[6] On the whole, this proposition has not been fully explored. The academic discourse and judicial consideration of the matter fail to consider policies which can supplement the patent regime and in effect dispel the ethical fears related to patenting of methods of medical treatment. In particular, the government’s subsidisation policies in relation to health care assist in ensuring that the Australian public is provided with the same level of universal access despite the existence of patented methods of medical treatment.
The relevant subsidisation policies are embodied in Medicare including the Pharmaceutical Benefits Scheme (‘PBS’), the Private Health Insurance Rebate and Commonwealth government grants. These policies are analysed to determine if their scope is such to accommodate patented methods of medical treatment and ensure access to health care despite the existence of such patents. The adequacy of funding arrangements and the level of health costs are examined to determine their sustainability in light of patented methods of medical treatment, along with their ability to redistribute income. Lastly the effect of proposed changes on the current arrangements are examined to determine if these will improve or worsen the situation.
This investigation also has implications for the role of ethical arguments in patent law. Ethical theory often engages in discussion of the appropriateness of laws and it can inform judgments through the avenue of public policy considerations. If the conclusion can be drawn that subsidisation and related government policy is adequate to maintain access to healthcare, then the ethical concerns raised in this respect lack plausibility and their usefulness should be questioned. It would also indicate that the court is correct in not forming legal judgments on such a basis.[7]
Under international law, it appears certain that methods of medical treatment are a proper subject matter for patents. Article 27 of the Trade-Related Aspects of Intellectual Property Rights Agreement (‘TRIPS Agreement’ or ‘TRIPS’) of the General Agreement on Tariffs and Trade provides that ‘patents shall be available for any inventions, whether products or processes, in all fields of technology, provided they are new, [are non-obvious] and are capable of industrial application’.[8] Therefore the treaty is wide enough to include methods of medical treatment as patentable subject matter. However, Article 27.3(a) of the TRIPS Agreement does allow member states to exclude from patentability ‘diagnostic, therapeutic and surgical methods for the treatment of humans or animals’.[9] The Australian government dealt with the issue in only a limited sense with s 18(2) of Patents Act 1990 (Cth), which states that ‘human beings and the biological processes for their generation are not patentable inventions’. Thus, in Australia there is no direct statutory bar to the granting of patents over methods of medical treatment.[10]
For decades, the subject matter of methods of medical treatment was not treated as being susceptible to patenting due to an exclusion which had developed through case law.[11] However, judicial opinion on the subject of patentability of methods of medical treatment was liberalised when the scope of manner of manufacture (and thus patentable subject matter) was broadened in National Research Development Corporation v Commissioner of Patents.[12]
The major breakthrough in terms of judicial reasoning was made in Anaesthetic Supplies Pty Ltd v Rescare Ltd[13] (‘Anaesthetic Supplies’). This case concerned the validity of a patent granted over a method for treating snoring and/or obstructive sleep apnoea.[14] The patent was held to be invalid by the whole court, on the grounds that it was not fairly based.[15] However, Lockhart and Wilcox JJ, expressed the opinion that there was no legal constraint upon the court recognising the validity of granting a patent over a method of medical treatment.[16] Justice Lockhart stated that there was no justification in law or in logic to draw a distinction between a substance that produces a cosmetic result as opposed to a curative result,[17] thereby rejecting such a distinction as representing a form of exclusion.[18] Further, his Honour stated that once the notion of vendible product is removed, as it was in National Research Development Corporation,[19] there is no distinction between a substance for treating and a method for treating the human body.[20] Justice Wilcox agreed generally with Lockhart J, and further stated that since there had never been a decision of an Australian court against such patentability,[21] and since the legislature had not been persuaded by any policy consideration, the legislative silence on this point can be interpreted as deliberate.[22]
The decision of the Federal Court in Bristol-Myers Squibb Co v FH Faulding & Co Ltd[23] (‘Bristol-Myers’) seems to have provided some consistency and stability to this area of the law by confirming the earlier Federal Court decision in Anaesthetic Supplies. Justices Black and Lehane felt they were bound by this earlier decision and based their decision on two further considerations. First, that it would be illogical to draw a distinction between a product for treating the human body and a method of treatment of the human body which brought about the same beneficial result.[24] Secondly, their Honours saw it as pertinent that the legislature had only dealt with patents in respect of the human body to a limited extent and that it had been the practice of the Australian Patents Office to regularly grant patents over methods of medical treatment.[25] In particular, their Honours agreed[26] with Justice Lockhart’s statement in Anaesthetic Supplies that the parliament had the opportunity to legislate in relation to the patentability of methods of medical treatment, with the authority of the TRIPS Agreement, but it failed to do so.[27]
In order to understand the nature of the ethical concerns that have been raised in relation to methods of medical treatment, it is useful to consider the types of methods or processes for which patent applications have been filed. I conducted a search of the Published Patent Data Searching Database to discover the types of claims being made in Australia.[28] The first 1000 documents returned by a search under the term ‘method’ in the title field revealed that 33, or 3.2 per cent, of these applications were made for a patent over a method of medical treatment.[29] Of these applications, 28 were made under the Patent Cooperation Treaty[30] (‘PCT’) and were thus overseas applicants, that is, approximately 88 per cent of these applications were foreign.[31] These results are consistent with general patent application activity, which indicate that in 1991 approximately 91 per cent of all patents granted in Australia belong to non-residents.[32] This reflects a long-standing trend, with similar low domestic statistics being recorded in the 1960s and 1970s.[33] In 1999 to 2000 residents of the United States alone obtained, in Australia, more than twice as many standard patents as Australian residents.[34]
Of the 32 applications, four related to methods of treating cancer.[35] The search also uncovered an application made in relation to a method for treating diabetes by pharmaceutical agent.[36] Another application included a method for treating action tremor or severe essential tremor.[37] The search results also included applications to patent a method of treating degenerative bone diseases such as osteoporosis, Paget’s disease and Gaucher’s disease,[38] a method for treating tumours by reducing white blood cells and inhibiting CD promoters[39] and a method of treating airway disease conditions by inducing, stimulating or otherwise facilitating bronchoprotection in the patient.[40]
The above examples assist in illustrating why this is such a poignant subject. For instance, in 1999 cancer accounted for 29 per cent of male deaths and 25 per cent of female deaths in Australia.[41] However, survival rates after treatment are quite high with on average 56.8 per cent of males and 63.4 per cent of females surviving cancer.[42] Thus the effect of a restriction on access to a method of treatment of cancer, such as the pending applications mentioned above, could cause unnecessary death because effective treatment would exist but not be available to those affected by the disease. The beneficial effect which these methods of treatment would have on the quality of life of Australians also highlights the importance of determining if patents over methods of medical treatment would actually limit access to health care.
A number of limitations are faced when assessing the impact which methods of medical treatment will have on the cost of health care. These include determining how many patents have been granted for methods of medical treatment, how crucial those patented treatments are to human welfare, the cost of royalties, and whether the patentees are enforcing their rights. In Australia, no such data has been collected. Figures estimated in the United States may provide a guide, however methods of medical treatment have been patentable longer there than in Australia.[43] By 1994, it was estimated that the Patent and Trade Mark Office in the United States was approving approximately 12 medical procedure patents per week[44] and in 1996 it was estimated it was approving on average 100 medical procedure patents per month.[45]
The controversy surrounding the patentability of methods of medical treatment arises from the competing forces of economic theory, which underpins patent law, and the ethical concerns regarding the effect of patent law on public welfare. In particular, the tension arises because of a perception that patent law tends to allow the economic interests of large companies to supersede the interests of the greater population.
The judgments of Finkelstein J in Bristol-Myers and Sheppard J in Anaesthetic Supplies exemplify the types of ethical concerns that patents raise over methods of medical treatment. These judgments therefore form appropriate benchmarks for an analysis of current concerns. In summarising the arguments against the grant of a monopoly over methods of medical treatment, Finkelstein J arranges the main contentions into two broad groups.[46] The first is the adverse effects on the provision of health care.[47] His Honour was of the opinion that the most powerful argument against the patentability of methods of medical treatment was the possibility that patients may be denied necessary medical treatment either because the medical practitioner does not have the license to use the patented method and fears an infringement suit, or because the patient cannot afford to pay the cost of the treatment, which has increased because it includes a royalty component.[48] Sheppard J argued that to grant a patent to one medical practitioner over a method of medical treatment which is greatly beneficial to the public could result in unnecessary death or pain.[49] Loughlan suggests that the reluctance to use patented methods of medical treatment could even extend to the use of similar methods to those patented.[50] Not only is it true that this argument is perhaps the most emotive of the ethical arguments, it may also be the most important ethical concern because if such a state of affairs eventuated, it would directly affect Australians’ health and government expenditure.[51] When determining the question of whether methods of medical treatment should be patentable, the concern that patients may be denied access to health care is thus the most frequently raised ethical issue.[52]
In Australia, to date, there have been no reported instances of action brought against a medical practitioner for infringement of a patent over a method of medical treatment. However, such a case has arisen in the United States and serves as an example of how such patents can affect access to health care. The case of Pallin v Singer[53] is thought to be the first infringement action in which one doctor sues another for infringement of a pure method of medical treatment patent.[54] Dr Pallin sued Dr Singer for performing his patented sutureless cataract procedure without a licence.[55] Although Dr Pallin claimed that he was only seeking royalties from other doctors in the range of three or four dollars per operation,[56] even such nominal royalties could add millions to the national cost of health if the patented method concerned were a popular method of medical treatment.
In the United States, it has been suggested that patents over methods of medical treatment may increase costs of health care not just through royalty payments but also by legal action taken to protect patent rights.[57] This is due to the fact that doctors who patent methods of medical treatment are usually more interested in licensing the method and collecting royalties than in restricting access to that method.[58] Disputes concerning the validity of patents over methods of medical treatment or legal action to enforce the right to collect royalties, could result in millions of dollars being spent on legal fees.[59] For example, in Pallin v Singer, the plaintiff’s legal costs were almost US$500 000.[60] These legal costs would eventually be passed on to the patient.[61] It may be that the cost of litigation for the infringer could be covered by medical indemnity insurance, however the legal fees of the patentee may well be recovered through higher royalties.
The second broad category identified by Finkelstein J related to adverse effects on medical progress and education.[62] In Anaesthetic Supplies, Sheppard J concentrated on the effect that patents could have on the dissemination of knowledge in research communities.[63] Justice Finkelstein took this argument further in Bristol-Myers by suggesting that due to the potential that researchers may deliberately withhold research results and medical knowledge, a patent may actually act as a disincentive to further invention.[64] In Pallin v Singer, Dr Singer wrote an article which was published in an academic journal informing colleagues of the sutureless cataract procedure that he believed he had discovered.[65] This article led to Dr Pallin’s discovery that his patent had been infringed. This case demonstrates how patents may deter doctors from sharing their innovations and discourage inventiveness.
Generally, the main justification for patent monopolies is that they provide an incentive to innovate. It is also asserted that patent law represents a compromise between innovation and monopolies over useful technology, with the purpose of ‘securing future benefits for the common good’.[66] However, the empirical research conducted in relation to the effect of patent protection on innovation has produced mixed results.
A 1973 study in the United Kingdom, by Taylor and Silberton, found that private research and development was not dependent on patent protection and thus concluded that patents are not relevant to innovation.[67] A similar survey conducted in the United States by Mansfield found that approximately half of the patented inventions would not have occurred without patent protection, but there was a strong bias towards pharmaceutical and chemical companies.[68] Other research in the United States by Levin, supported Taylor and Silberton’s findings.[69] Research in Australia indicates that patents have a greater effect on innovation than the results of Taylor and Silberton would suggest. In a study by Mandeville, Lamberton and Bishop in 1982, 50 per cent of firms indicated that their research and development activities would decrease if there was no patent protection in Australia.[70] Moreover, 60 per cent of participants indicated that patent protection increases sales and profitability. Therefore, at least in Australia there appears to be some empirical support for the assertion that patents do promote innovation.
However, it is argued that there is little evidence to suggest that the innovation incentive offered by patent protection is required for doctors to have the motivation to research medical methods or share their discoveries.[71] The development of new methods of medical treatment often occurs during the normal course of the doctor’s practice.[72] Therefore, there is little requirement for the levels of capital investment required with, say, pharmaceuticals.[73] In spite of this, Lockhart J in Anaesthetic Supplies was of the opinion that reasons for granting patent protection for methods of medical treatment had been substantiated, because denying protection would result in a reduced incentive to spend time and money to make such discoveries.[74] Another commentator argues that excluding methods of medical treatment from patentability will undermine the certainty of the patent system and thus lessen the general innovation incentive offered by patent law.[75] Patent protection for methods of medical treatment has also been justified on the basis of Australia’s status as a net importer of technology.[76] From this, the inference can be drawn that failing to provide such protection could result in Australia missing out on the benefits of overseas research and development.[77]
The current Australian academic and judicial discourse fails to consider all aspects of the ethical issues raised concerning the patenting of methods of medical treatments. In particular, commentators generally assume that there will automatically be a negative effect on the provision of health care if methods of medical treatment continue to be patentable. That is, ethical arguments in this area of the law tend to disregard the role which patents play in the Australian economy, the effect of patents on economic activity, and the subsidisation policies of the government in relation to health care, or other policies that could be put into place to prop up access to health care.[78]
Judicial consideration of the economic impact of patented methods of medical treatment and the national interest in retaining access to health care has been limited. In Joos v Commissioner of Patents,[79] Barwick CJ pointed out there is a national economic interest in ‘repair and rehabilitation of members of the workforce’[80] and that ‘one has only to recall the economic impact of workers compensation, invalid pensions and repatriation costs to recognise’[81] the proximity between good health care and economic prosperity of a country. In Aktiebolaget Hassle v Alphapharm[82] Kirby J alludes to the high costs which patent protection can cause for the Australian government in the area of health care. The pharmaceutical compound which was the subject of that case had already enjoyed a twenty-year monopoly and in one year of being listed on the Pharmaceutical Benefits Scheme had cost the government over A$141 million, a cost ultimately borne by Australian taxpayers.[83] In Commissioner of Patents v Wellcome Foundation Limited,[84] Cooke J makes the observation that the appropriateness of patenting methods of medical treatment must be considered in the light of economic questions important to a country the size of New Zealand, heavily reliant on overseas manufacturers.[85] This issue should be relevant to Australia’s considerations because Australia is also an importer of technology. Only 9 per cent of Australian patents originate from Australian residents.[86]
The issue of patenting of methods of medical treatments has been largely ignored in terms of national enquiries and research papers. The extent of formal enquiry into the issue is a discussion paper examining the effect that gene patents may have on the provision of health care, prepared by the Australian Law Reform Commission.[87] The paper focuses on the effect of genetic diagnostic testing which is arguably not a method of medical treatment because it occurs before treatment is received or given.
In assessing whether government subsidisation policies would be adequate to deal with patented methods of medical treatment, it is necessary to consider two issues. First, the manner in which Government subsidisation policies ensure access to healthcare. Second, the cost of health care in Australia needs to be analysed to determine the ability of the system to cope with increased costs and to determine if there has been any observable impact on health care costs caused by the introduction of patented methods of medical treatment.
There are three main methods of subsidisation of hospital and public health services provided by the government at present: Medicare, which includes the PBS; incentives to obtain private health insurance; and specific federal government grants. These forms of subsidisation could assist in ensuring equitable access to healthcare in the face of patented methods of medical treatment.
The Australian Health Care Agreements, between the Commonwealth and State and Territory Governments, specify the functions of each of these parties in relation to the provision of health care in Australia.[88] The Commonwealth Government has adopted a leadership role in policy-making and in national issues such as public health and research.[89] It also funds out-of-hospital medical services and most health research.[90] The States and Territories are charged with responsibility for the delivery and management of public health services.[91] The Commonwealth, State and Territory Governments jointly fund public hospitals and community care for aged and disabled persons.[92]
The agreements are premised on the idea that public hospital services should be provided free of charge to public patients, on the basis of clinical need, within an appropriate timeframe, and that people should have equitable access to public hospital care regardless of their geographic location.[93] This appears to be a long-term and continuing goal, suggesting that the government will ensure that there is continued equitable access to health care in the face of patenting of methods of medical treatment.
The major form of subsidisation of the health care system is Medicare. All individuals eligible[94] for Medicare are entitled to free accommodation and medical, nursing and other care as public patients in State and Territory owned hospitals and some private or charitable hospitals.[95] Private doctors’ services, pathology tests and some optometric services and dental surgery are generally reimbursed either fully or in part by Medicare benefits.[96] Medicare benefits may be claimed for items which are listed on the Medicare Benefits Schedule, and a rebate is payable up to the amount of the listed schedule fee applicable for each item.[97] Doctors may charge any fee they wish, provided the service is not bulk-billed, and in such a case the rate of benefit is at least 85 per cent of the Medicare Benefits Schedule fee.[98] For individuals who choose to attend a hospital as a private patient the rate of Medicare benefit that can be claimed is 75 per cent, and the private health insurer covers the 25 per cent gap in cost.[99]
The types of medical treatments listed on the Medicare benefits schedule are expansive, and most major diseases and treatments are listed. Listed treatments include radiation oncology treatment used to treat cancer,[100] total mastectomy of female breast often used to treat breast cancer,[101] treatment of premalignant skin lesions used to remove moles and freckles at risk of becoming malignant,[102] and Coronary Artery Bypass procedure.[103] Thus it would be possible for a patient to claim a Medicare benefit for a patented method of medical treatment, provided it was listed on the Medicare Benefits Schedule, and pay no fee for the treatment or at least receive a rebate.
As a further protection against excessive health costs to a patient, there is a safety net in place that applies when a patient or family receives many services in one year and the patient’s ‘gap’ payments in that year exceed a threshold amount. All further benefits in that year are then paid at up to 100 per cent of the schedule fee.[104]
The PBS is a large part of Medicare. The aim of the PBS is to provide all persons eligible for Medicare with access to necessary prescription medications at a reasonable cost.[105] It is estimated that approximately 90 per cent of all prescriptions dispensed in Australia are for pharmaceuticals subsidised by the PBS[106] and in the year ended June 2002 benefits paid totalled A$5003 million.[107]
The PBS subsidy is only provided for drugs which are approved for listing on the Pharmaceutical Benefits Schedule. Eligible patients are divided into two groups as general and concessional patients, with different benefits conferred to each group. Both categories of patients pay the cost of the prescription medicine to a pre-determined maximum amount per item and the government covers the balance of the cost of the drug.[108] The maximum amount payable by concessional patients is less than general patients and thus the PBS provides a larger subsidy per item for the concessional category consumers.[109] Overall the subsidy rate for PBS listed pharmaceuticals for concessional patients is 90.2 per cent, whereas it is 65.4 per cent for general patients.[110] This is substantial, as on average concessional patient prescriptions comprise 80 per cent of total government expenditure on the PBS.[111]
The PBS also provides safety net provisions which state that patients whose payments for PBS listed medicines reach a certain threshold in a calendar year pay a reduced amount from that time to the end of the year. For concessional patients no further payment is required once the threshold is reached.[112]
The PBS is important for maintaining access to methods of medical treatment which prescribe a particular use of a patented drug. In Bristol-Myers, the method of medical treatment in question was a method for treating cancer which specified the dosage and method of administering the drug Taxol.[113] In this case it is questionable whether access to the method of treatment would be greatly restricted due to patentability because the drug Taxol is listed on the PBS Schedule and therefore is subsidised.[114] When a patented method of medical treatment is attached to a pharmaceutical, a user of the method of treatment would need to purchase the drug from the patent holder or licensee in order to comply with the patent.[115] Thus the royalty payment for the patented method of medical treatment is comprised in the price of the pharmaceutical.
Consequently, in Australia, the PBS is already assisting in ensuring access to patented methods of medical treatment. This is because some of the pharmaceuticals listed on the PBS, and thus attracting the subsidised price, would include methods of medical treatment covered by patents. The effect is substantial considering that of the 32 patent applications relating to methods of medical treatment found in my search of current patent applications, 21 related to therapeutic compositions. The PBS can therefore perform the dual function of suppressing pharmaceutical prices and the ‘royalty’ price of patented methods of medical treatments attached to pharmaceuticals listed on the Pharmaceutical Benefits Schedule.
The PBS seems to be effective in ensuring that access to health care is retained by the people most at risk of being denied access due to cost barriers. A recent study of the distributional impact of the PBS has concluded that the scheme is essentially aimed at assisting the poorest sections of the community in accessing necessary medications.[116] The results showed that the poorest fifth of all Australians received an average PBS subsidy of A$8.70 per week compared with A$1.60 per person for the fifth of Australians in the top ranking income bracket.[117] Overall, the PBS tends to provide the greatest benefit to the elderly, which is the segment of the community with the poorest health,[118] and to those with the lowest income, the poor.[119]
The PBS is not only important for protecting access to health care by ensuring affordability of medication. Its success in so doing also means that it can provide a model for a potential scheme to contain royalty payments relating to patented methods of medical treatment. For instance, the government could use Medicare to contain the costs of patented methods of medical treatment in much the same way that the PBS is used to contain the cost of pharmaceuticals.
The government has at its disposal two main methods of exerting buyer bargaining power in order to lower the price charged by pharmaceutical companies.[120] First, applicants for the PBS must demonstrate clinical advantages and satisfactory cost effectiveness compared to alternative drugs. The Pharmaceutical Benefit Pricing Authority uses this comparative pricing as leverage in negotiations with the applicant.[121] Secondly, companies are forced to justify the listing and price of an innovative drug through economic and therapeutic studies that show that a clinical advantage exists over the main competitor.[122] Further, the fact that the technology used in pharmaceuticals allows for statutory protection via patents, implies that prices will not be fixed, but will instead depend on demand, creating a source of bargaining power for the Australian government.[123] Pharmaceutical companies are forced to bargain with the Australian government to obtain listing on the PBS and are prepared to trade off a lower price for the volume benefits of listing.[124] Therefore pharmaceutical companies are forced to provide drugs at a lower price in Australia than they ordinarily would. A similar scheme for patented methods of medical treatment could operate in a similar manner to the PBS, with the government exerting its buyer bargaining power to reduce prices in exchange for a patentee gaining listing of a method of medical treatment on the Medicare Benefits Schedule.
The government provides a 30 per cent subsidy, in the form of a rebate, to persons who obtain private health insurance and has also introduced a program to encourage life long membership.[125] After 1 July 2000, people who are not members of a health care fund by the time they are 30 years old, will be charged an extra 2 per cent of the base rate, which is the standard price that an individual health fund charges for hospital cover in addition to the normal premium, for each year they are aged over thirty, until they choose to join a health fund.[126] This provision has caused an increase in the level of coverage from 32.2 per cent in March 2000 to 45.8 per cent in September 2000.[127]
At present, private health funds offer hospital cover, ancillary services cover or a combination of these. Ancillary services include services provided by private dentists, physiotherapy, chiropractic treatment and aids such as contact lenses.[128] Hospital cover can cover all or some of the costs of hospital treatment as a private patient in both public and private hospitals and includes choice of doctor as a private in-patient.[129] Basic hospital cover will usually cover private hospital accommodation, intensive care (includes coronary unit care), theatre fees and same day accommodation.[130]
Individuals with private health insurance still retain their Medicare entitlements and are able to use public hospitals as either Medicare patients or private patients.[131] The difference is that when a person is admitted as a private patient in either a public or private hospital, Medicare pays for 75 per cent of the Medicare Benefits Schedule fee for the doctor’s fees for in-hospital services and the health fund pays the remaining 25 per cent.[132] If an insured patient is admitted as a public patient to a public hospital, Medicare will pay either 85 or 100 per cent of the Medicare Benefits Schedule fee.
The theory behind the government’s subsidisation of private health insurance, rests on the idea that eventually some of the strain on the government to fund health care will be relieved if enough of the population is covered by health insurance. Nevertheless, it may be more economic for a private patient to be admitted as a public patient to receive Medicare Benefit Schedule listed treatments, depending on their type of cover. For example, a person may choose to pay an excess or co-payment for treatment received in return for a reduced premium, and could avoid incurring the excess or co-payment by being admitted as a public patient. In some cases a reduced premium can also be attracted by obtaining cover with restricted treatment of service which provides only limited benefits for certain treatments, such as in-hospital psychiatric treatment or infertility treatments.[133] Often restricted benefits cover will provide greater benefits if the restricted treatment is received in a public hospital rather than a private hospital.[134] Obviously, if the treatment received is not listed on the Medicare Benefits Schedule then a Medicare rebate will not be attracted and it would be preferable to claim private health insurance benefits.
Because persons holding private health insurance can still be admitted as Medicare patients, it is not entirely clear that private health insurance would assist in alleviating the cost of royalty payments for using a patented method of medical treatment. There has been little data collected on the rate of insured patients who choose to be admitted as public patients. However in 1999 a study estimated that in 1995–96, 33 per cent of insured patients admitted to public hospitals were admitted as public patients, whereas 67 per cent chose to be admitted as private patients.[135] These results tend to suggest that on average most holders of private health insurance choose to claim their benefits when receiving treatment at public hospitals.
Traditionally more complex treatments have only been available in large public hospitals.[136] However, increasingly, private hospitals are widening the types of services provided. Intensive care, cardiac surgery, neurosurgery, renal dialysis and oncology are among the services now being provided.[137] High cost procedures such as bone marrow transplants and tracheostomies are also now being conducted in private hospitals.[138] In 1993–94 there were 10 bone marrow transplants reported for private hospitals but by 1999–2000 this had increased to 200.[139] The implication of providing greater services is that private hospitals can alleviate some of the strain on public hospitals and thereby improve access to healthcare. An indication of this shift is that in 1994, 32 per cent of coronary bypass grafts were provided in private hospitals and this increased to 41 per cent in 2000, whereas in the public sector the number of coronary artery bypass grafts decreased by 6 per cent for the same period.[140] Further, increased services imply that increased use of private hospitals can be made by insured patients and, along with the increase in coverage, can indicate that private health insurance will increasingly cover the cost of a method of medical treatment, whether patented or not.
The third form of subsidy is the provision of a range of grants by the Commonwealth to the government and non-government entities, under the scope of Medicare, to achieve specific health care objectives.[141] Such objectives include the provision of services to special needs groups and funding medical services involving use of expensive equipment.[142]
With or without patenting of methods of medical treatment, no state can afford to provide all available forms of health care to its citizens. A French study published in 1973 researched what it would cost to provide all health care that would be beneficial to each citizen, and found it would cost five and a half times the Gross National Product of France.[143] This implies that health care must be rationed in a society to retain viable access.[144] In Australia, rationing of healthcare services comes in the form of subsidised medical services and the government has chosen the most beneficial services that all of society should have access to. Such a system is ethical because it excludes certain procedures from universal access, rather than excluding persons from the system.[145]
However, access to health care can be hampered by medical method patents if funding of the health care system is inadequate to keep up with rising costs, or if the system fails to redistribute income to the disadvantaged.
The structure of health care funding in Australia causes a favourable redistribution of income, protecting access to health care for people on lower incomes. Approximately 27 per cent of total Commonwealth funding for Medicare is collected through the Medicare levy.[146] The Medicare levy is paid by individuals who earn over a certain threshold amount at a rate of 1.5 per cent and high income earners who do not have private health insurance pay an additional 1 per cent.[147]
In a study by the National Centre for Social and Economic Modelling at the University of Canberra, it was found that Medicare, through the Medicare Levy, does tend to redistribute wealth so that universal access to health care is achieved.[148] The results of the study indicated that the health needs of lower and middle income groups are cared for without placing a large cost burden on these income groups.[149] People in middle and low income groups are most likely to receive public benefits through hospitalisation, report poorer health and are older than higher income groups.[150] They are therefore more likely to be affected by an increase in the price of medical treatment. The upper income group bears most of the burden of the Medicare levy, raising 60 per cent of its total revenue. This group does receive some benefit from their contribution, although it is less than the amount they pay through the Medicare levy.[151] On average the upper income group paid A$108 a year in Medicare levy more than they received in-hospital benefits.[152] The authors of the study concluded that the public expenditure on hospitals is considerably ‘pro poor’.[153]
The current funding pattern of health care appears, at least in terms of hospital care, to be adequate for accommodating the health care needs of the people most in need of medical attention, that is, people in low income groups. In light of this research, it seems likely that current funding patterns are enough to ensure that equitable access to medical treatment is retained, even if a royalty were charged by a patentee.
One inherent characteristic of a patent is that it allows its owner to charge monopoly prices. This point is often used to argue that patents over methods of medical treatment will necessarily drive up the cost of health care and that such an increase cannot be borne by the Australian economy in the face of ‘spiralling health costs’. However such claims are often unverified, instead reflecting a public perception of health care shaped by constant bombardment of media reports stating that health care in Australia is in dire straits. This perception may not be accurate, as such information mediums are often used to promote particular platforms rather than the actual state of affairs.[154] The Doctors Reform Society argues the claims that health costs are spiralling out of control are unsubstantiated.[155] Rather, statistics show that growth in health care costs has increased at a stable rate. In 2000–01 Australia spent an estimated A$60.779 billion on health care and A$66.6 billion in 200 1–02.[156] This amounts to 9.1 per cent of the GDP in 2000–01 and an estimated 9.3 per cent of the GDP in 200 1– 02.[157] The annual growth in this percentage has been stable since the early 1990s. This tendency is illustrated in the table below.
TABLE 1: TOTAL HEALTH EXPENDITURE AND GDP, CURRENT PRICES AND ANNUAL GROWTH RATES, 1990–91 TO 2000–02
|
Year
|
Total Health Expenditure
|
GDP
|
Ratio of
Health
Expenditure
to GDP
(%)
|
||
|
Amount
(A$m)
|
Nat’l (%)
Ann.
Growth
Rate
|
Amount
(A$m)
|
Nominal
(%) Ann.
Growth
Rate
|
||
|
1991-92
|
33 123
|
5.9
|
406 605
|
2.2
|
8.1
|
|
1992-93
|
35 098
|
6.0
|
426 231
|
4.8
|
8.2
|
|
1993-94
|
36 990
|
5.4
|
447 024
|
4.9
|
8.3
|
|
1994-95
|
39 216
|
6.0
|
471 349
|
5.4
|
8.3
|
|
1995-96
|
42 082
|
7.3
|
502 828
|
6.7
|
8.4
|
|
1996-97
|
45 296
|
7.6
|
529 885
|
5.4
|
8.5
|
|
1997-98
|
48 278
|
6.6
|
561 229
|
5.9
|
8.6
|
|
1998-99
|
51 629
|
7.0
|
591 916
|
5.5
|
8.7
|
|
1999-2000
|
55 809
|
8.1
|
628 620
|
6.2
|
8.9
|
|
2000-01
|
60 897
|
9.1
|
669 307
|
6.5
|
9.1
|
|
2001-02[*]
|
65 582
|
9.3
|
712 874
|
6.5
|
9.3
|
Source: Australian Institute of Health and Welfare, Health Expenditure Australia 2001–2002, Health and Welfare Expenditure Series No 17 (2003) 9.
[*] Based on estimates.
Health cost prices have also remained stable. In the periods 1999–00 and 2000–01 the growth in health cost prices was 5.1 per cent, which was close to the eight-year growth rate average since 1992–93.[158] Figure 1 reflects the pattern of stability in total health expenditure and annual growth rates. However, over the last decade the health sector of the economy has grown faster than the economy as a whole.[159] Yet this may not be a cause for concern, because the GDP in real terms has increased by approximately 4.1 per cent with the health cost component rising by 4.6 per cent.[160] So, health care costs have only exceeded the general rate of economic growth by 0.5 per cent over a period of 10 years, which counters claims of ‘spiralling health costs’.[161]
FIGURE 1: COMPARISON OF HEALTH EXPENDITURE AND ANNUAL GROWTH AT CONSTANT PRICES, 1990–91 TO 2000–01
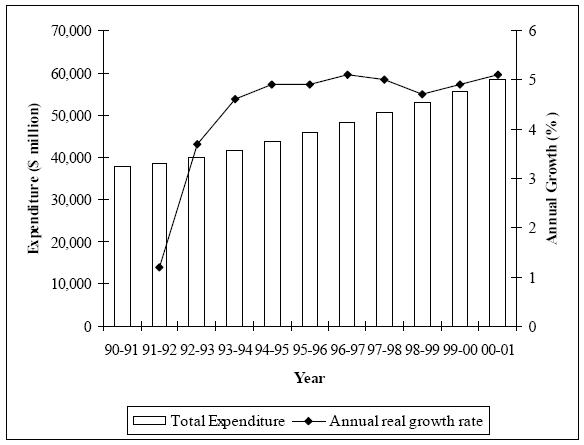
Source: Australian Institute of Health and Welfare, Health Expenditure Australia 2000–2001, Health and Welfare Series No 14 (2002) 8.
The effect of the cost of patented methods of medical treatment on overall health costs may be difficult to detect, because such patents have only clearly been patentable in law since 1994. However it is reported that the Patent Office had a long-standing policy of accepting such patent applications,[162] meaning that patented methods of medical treatment probably existed long before 1994. Any negative effect, at least of the magnitude argued by advocates of an exclusion, would be likely to have transpired by now, but there is no clear detriment to be observed. The Australian Institute of Health and Welfare indicates that any increase in health expenditure to GDP, which has been seen in recent years, can be blamed on two causes: either the level of use of goods and services in health increased at a greater rate than the increase in the use of all goods and services in the economy,[163] or the rise in prices in the health sector was greater than the rise in prices economy wide, often referred to as excess health inflation.[164] A major part of this increase in health expenditure has been a result of population growth, as it is logical that as the population increases expenditure must also increase to maintain the same average level of goods and services.[165] This is reflected in the figures showing that real growth in health expenditure per capita, from 1990–91 to 2000–01, averaged 3.2 per cent, compared to an average growth rate of 4.4 per cent for aggregate national health expenditure for the same period. The difference in these two growth rates is caused by an increase in the overall size of the population.[166] The two areas which experienced the most growth in usage by the population were the hospital and pharmaceutical sectors, which form two of the major areas in which patented methods of medical treatment operate.[167]
Most recently, rises in health expenditure have been caused by increases in the population rather than increases in prices, suggesting that patented methods of medical treatment have not caused the price of health care to dramatically increase. Further it could be assumed that somewhere along the track these increased costs will be recouped from the increased tax payments which will be made by the larger population. Overall, the annual increase in health care expenditure has been able to remain gradual in the face of the supposed inflationary effects of patented methods of medical treatment.[168]
The current health care subsidisation policies implemented by the government appear to be fairly adequate in deflecting any negative effect on health care which patented methods of medical treatment are believed to cause. However, the balance between government subsidisation and patent monopoly may not be sustainable if existing policies are changed. In particular, there have been amendments proposed for Medicare and international pressure has threatened the viability of the PBS.
The government has launched a proposal to amend Medicare, through the package ‘A Fairer Medicare – Better Access, More Affordable’.[169] Under the proposed changes concession cardholders[170] will be eligible to receive medical care at no cost from doctors participating in the scheme.[171] Non-cardholders attending practices participating in the scheme, but which do not bulk-bill, will be required to only pay the difference between the Medicare Schedule fee and the doctor’s fee, rather than having to attend a Medicare office to claim the rebate.[172] For concession cardholders with high cumulative out of pocket expenses, safety nets will be strengthened. The safety net will include a wide range of Medicare services and will cover the entire fee paid to doctors, rather than just the Medicare Schedule fee amount, as is now the case.[173]
Safety nets for other patients would not be strengthened but instead, a new private health insurance cover will be made available independent of other private health products. This new coverage will be able to offer 100 per cent cover for total out-of-pocket costs for Medicare services received out of hospital of more than A$1000 in one year.[174] This suggests that the government intends to rely more heavily on private health insurance to lighten the burden of national health costs and at the same time provide further incentive for Australians to obtain health cover.
The suggested amendments attempt to redistribute even more wealth to the disadvantaged in our society, that is, the elderly, sick and poor, who are typically cardholders. Therefore it appears that the new scheme will probably improve access to health care. However there are two issues which arise from these changes. Firstly, by shifting more of the wealth from the high income earners to the poor (through the Medicare Levy), these high income earners may end up receiving no benefit relative to the tax they pay. Under the proposed system, a greater share of the cost is also shifted to the middle class of income-earners, who are not entitled to health care cards and would be required to incur additional expense by obtaining health insurance to obtain the same safety net as received by cardholders. This would not make the scheme ‘fairer’, as the scheme’s title claims, but would shift a greater burden onto middle and high income earners. Bearing such disproportionate costs in relation to benefits received could be inequitable in the future.[175]
Secondly, complaints against insurers have steadily increased, raising issues that need to be addressed if the government intends to place a greater reliance on private health insurance. In the first quarter in 2004 there were over 737 complaints made about health funds, and 300 of those complaints related to benefits.[176] In light of this, the proposed new form of health cover may assist in appeasing some of these problems, covering out-of-hospital Medicare gap payments in relation to specialist treatment, x-rays, ultrasounds and radiation oncology, which are often high.[177] However, many of the health fund members who have made complaints do so in relation to substantial gap payments associated with specialist services in-hospital, even when covered by top-level hospital cover.[178] This is an issue which the amendments fail to address. Other issues consumers complained of included the attitude of insurers when making a claim, hidden costs, and inadequate notice of changes to terms of the policy.[179]
Many patients around the world are denied access to new pharmaceuticals because of the high price charged by the patent holder or their licensee.[180] Perhaps experiences with pharmaceuticals is a reason why many commentators are so adamant that methods of medical treatment should not be patentable, in case the same access problems recur.[181] Continued access to healthcare in Australia, in light of the fact that methods of medical treatment are patentable is in part dependent, by analogy to the position regarding pharmaceuticals under the PBS,[182] on the extent to which the government can use its purchasing power to negotiate favourable prices or is willing to subsidise such treatment out of public funds.[183] If the PBS no longer becomes viable, then by analogy any similar scheme for patented methods of medical treatment will also be redundant.
The Australian government recently completed negotiations with the United States to establish a free trade agreement. The aim of the agreement is to reduce barriers to trade between the two countries, and the PBS featured as a controversial issue. Representatives of the US government, such as Under Secretary of Commerce Grant Aldonas, publicly called for the abolition of the PBS on the basis that it represents a barrier to trade.[184] However, the PBS has been retained and no changes were made to its actual structure but changes have been made to administrative aspects of the review process. These changes focus on shortening the time within which an application must be considered and improving transparency of the approval process by requiring the Pharmaceutical Benefits Advisory Board to provide reasons for rejecting a pharmaceutical for listing.[185]
The PBS has two main implications that irritate the United States and form the basis for their claim. First, the structure of the PBS and the approval process for obtaining listing are involved and comprehensive. In particular, the United States has complained that the effectiveness of the PBS in reducing prices of pharmaceuticals disadvantages research-based US pharmaceutical companies by tying the prices of innovative US drugs to the lowest priced medicines in their therapeutic groups. The American Pharmaceutical Research Manufacturers Association has also complained about the need to demonstrate sufficient clinical advantages, and the need to justify prices.[186] It is not surprising that such complaints would be made when most of the pharmaceuticals listed or attempting to be listed on the PBS originate from US-based or -owned pharmaceutical manufacturing companies. Combined with the large government subsidy attached to the PBS and the tendency of doctors to subscribe PBS listed drugs because of the cost advantage for patients, a company’s sales can be significantly damaged if it fails to gain listing of its pharmaceutical.[187]
Second, because the PBS reduces the price which Australians would ordinarily have to pay for pharmaceuticals, US pharmaceutical companies must recover the bulk of their development costs in the United States because it is the biggest and most lucrative market.[188] This is reflected in the fact that the proportion of elderly people who cannot afford to have a prescription filled is ten times higher in the United States than in Australia.[189] A discontinuation of the PBS would mean that Australia could contribute a greater share to recouping development costs by paying higher pharmaceutical prices. Thus the opposition of the United States to the PBS appears to be in the pursuit of national self-interest.
By contrast, it is not in Australia’s interest to align itself with the demands of more industrially-developed countries such as the United States, who have a national self-interest in strengthening intellectual property rights on a global basis, because such action will only damage Australia’s terms of trade due to its status as a net importer of technology.[190] Yet Australia’s heavy dependence on overseas trade and technology imports also means it is not in Australia’s long term interest to change or maintain the patents system in any way that contravenes international agreements and may therefore lead to political retaliations.[191] There is no legal impediment to the continuation of the PBS, as the scheme does not contravene the TRIPS Agreement. There is nothing in the TRIPS Agreement which prevents government funded subsidisation policies. In fact, TRIPS tends to recognise that intellectual property rights may limit access to public services. Article 8.1 allows member states, in formulating or amending their laws in accordance with TRIPS, to adopt measures to protect public health and nutrition.[192] Further, the TRIPS Agreement clearly states in Article 1.1 that members may, but are not obliged, to implement more extensive protection than is required by the TRIPS Agreement.[193] Thus, Australia is not under any international obligation to provide greater intellectual property rights than those required under the TRIPS Agreement and this reinforces the argument that there is no reason Australia should succumb to United States’ pressure to dismantle the PBS.
While it may be in the interests of the United States to discontinue or amend the PBS, it is not in the best interests of the Australian public. Nevertheless, for the time being it would seem that the PBS is not in any danger of being changed or removed. Former federal Minister for Health, Senator Patterson recently stated that the PBS ‘is a vital part of our health system’ and confirmed the government’s commitment to the PBS.[194] However, with strengthening diplomatic relations between the United States and Australia, there is always a danger that the PBS may be lost as a casualty to the cause of establishing closer trade relations between the two countries.
The issue of access to health care in Australia is not as bleak as some advocates for exclusion would have us believe. After an examination of subsidisation policies relating to health care, it would appear that these policies are likely to prevent access to health care being denied because of patenting, at least in the immediate future.[195] It is also possible that further protection could be provided by the use of Medicare to control the costs of patented methods of medical treatment in the same manner as the PBS, if the volume of such patents increased. However, in drawing this conclusion I do not wish to trivialise an important subject and despite this positive outlook there are further issues which need to be addressed.
There are two aspects of patents which could affect access to health care: royalties and infringement proceedings. Subsidisation policies address the issue of royalty payments, but the secondary issue of infringement proceedings against physicians has not been addressed by the Australian Government. From the American experience with methods of medical treatment, it can be observed that the real threat to access arises from the possibility of infringement proceedings, which prevents doctors from performing the patented method of medical treatment, and may entail expensive legal fees. This issue has not yet arisen in Australia, however I expect that it is only a matter of time before it does. This issue should be addressed by the government in a proactive manner, through pre-emptive legislative action. Legislation similar to that enacted in the United States would be appropriate. The amendments made in the United States protect medical practitioners who perform a medical activity as defined by the Act from infringement proceedings.[196] In addition, infringement proceedings relating to such activities may not be commenced against health care entities affiliated with that medical practitioner.[197] However, the legislation does not limit the ability of patentees to enforce patents against their competitors.[198]
Private health care will play an increasingly important role in ensuring access to health care in a marketplace in which methods of medical treatment are patentable. With the number of people taking out health insurance rising, there is normal health insurance covering all methods of medical treatment and a broadening in the scope of medical services provided by Private Hospitals. On the other hand, it is disconcerting that the trend in government policy is to place further reliance on the private health sector, when there is clearly consumer dissatisfaction with the services private health funds provide. Further, regulation is needed to address issues relating to the periods of notice required when a fund changes its policy and the accurate assessment of likely out-of-pocket expenses with gap payment cover. For private health cover to assist in ensuring access to patented methods of medical treatment, it must provide a feasible solution in practice and this means meeting customers’ expectations.
The fact that subsidisation policies are likely to be adequate to ensure access to medical treatment tends to suggest that ethical arguments are not a valuable basis for legal decisions in the area of patent law. Sweeping comments that ‘patented methods of medical treatment will deny access to health care’ are not substantiated and thus lack plausibility. Such arguments should therefore not be given substantial weight in the arena of judicial decision-making. There are so many issues and implications associated with the patentability of methods of medical treatment that a court would be unable to consider all the issues within the constraints of legal proceedings. This is particularly so since the negative effects of patentability are not clear nor evident in Australia and courts have traditionally shown a tendency to ignore Australia’s vulnerable position as a net importer of technology. Ethics do have a role to play in patent law, however their function should be limited to the process of legislative drafting and government lobbying. Parliament is the institution with the resources to deal with such public policy issues.[199] It is for the Government to determine whether the subsidisation policies in place are sufficient to support the additional costs which patents over methods of medical treatment may cause.
[*] B Bus, LLB (Hons) University of Technology, Sydney.
[1] In In the Matter of C & W’s Application for a Patent (1914) 31 RPC 235, 236, the Solicitor General found methods of medical treatment to be ‘lacking in commercial value’ and not associated with trade and commerce. More recently in Anaesthetic Supplies Pty Ltd v Rescare Ltd [1994] FCA 1065; (1994) 50 FCR 1, 18, Lockhart J acknowledged ‘the art of the physician or surgeon does not belong in the area of economic endeavour or trade or commerce’.
[2] ‘Medical treatment’ is defined by Barwick CJ as, ‘the purpose of the application to the body whether of a substance or process must be the arrest or cure of a disease or diseased condition or the correction of some malfunction or the amelioration of some incapacity or disability’: Joos v Commissioner of Patents [1972] HCA 38; (1972) 126 CLR 611, 619.
[3] Anaesthetic Supplies Pty Ltd v Rescare Ltd [1994] FCA 1065; (1994) 50 FCR 1; Bristol-Myers Squibb Co v FH Faulding & Co Ltd [2000] FCA 316; (2000) 170 ALR 439.
[4] Matthew Rimmer, ‘Myriad Genetics: Patent Law and Genetic Testing’ (2003) European Intellectual Property Review 20, 29. Section 6 of the Statue of Monopolies 1623, 21 Jac I, c 3 is sometimes considered to incorporate moral concerns into the patent process. However, no Australian case to date has excluded methods of medical treatment on the sole basis of being ‘generally inconvenient’.
[5] Australian Law Reform Commission, Gene Patenting and Human Health, Issues Paper 27 (2003) 62. This view is reflected in Australian Patent Office (‘APO’) practice. The Manual of Practice and Procedure states that matters of ethics or social policy are not relevant to deciding whether a subject matter is patentable and objections on this basis will not be accepted: APO, Patent Manual of Practice and Procedure Volume 2 – National (2002)[8.2.1] <http://www.ipaustralia.gov.au/pdfs/patents/manual/Part208.PDF> at 22 June 2004.
[6] Australian Law Reform Commission, above n 5. This view is supported by some commentators as an appropriate approach for dealing with social or ethical issues in this context. See, eg, Barbara Looney, ‘Should Genes Be Patented? The Gene Patenting Controversy: Legal, Ethical and Policy Foundations of an International Agreement’ (1994) 231 Law and Policy in International Business 101, 121.
[7] In relation to the patentability of medical and surgical procedures, see Bristol-Myers Squibb Co v FH Faulding [2000] FCA 316; (2000) 170 ALR 439. Finkelstein J concludes that, ‘the answer to this question cannot depend upon the resolution of moral or ethical issues … Judges should not be called upon to resolve moral questions and, speaking generally, legal principles should not be ascertained by reference to standards of ethics or morality’: at 472.
[8] Agreement on Trade-Related Aspects of Intellectual Property Rights, opened for signature 15 April 1994, 1869 UNTS 299 (entered into force 1 January 1995).
[9] Ibid.
[10] Patricia Loughlan, ‘Of Patents and Patients: New Monopolies in Medical Methods’ (1995) 6 Australian Intellectual Property Journal 5, 7.
[11] The exclusion was born out of the decision of Latham CJ in Maeder v Busch [1938] HCA 8; (1938) 59 CLR 684, which stated in obiter dicta that a mere method or process such as a method of medical treatment did not fall under the definition of ‘a manner of manufacture’: at 699. Although all four judges who heard the case, stated that the question of patentability of methods of medical treatment should not be decided in that case, later judgments interpreted the decision as creating an exclusion. The observations of Latham CJ were adopted in later cases such as National Research Development Corporation [1959] HCA 67; (1959) 102 CLR 252, 270 and Joos v Commissioner of Patents [1972] HCA 38; (1972) 126 CLR 611, 622–3.
[12] [1959] HCA 67; (1959) 102 CLR 252. The case held that it is not necessary to have a vendible product to obtain a patent, as long as the process produces a useful result. This decision was later used in Anaesthetic Supplies Pty Ltd v Rescare Ltd [1994] FCA 1065; (1994) 50 FCR 1 to dismiss the ‘exclusion’ created in Maeder v Busch [1938] HCA 8; (1938) 59 CLR 684.
[13] [1994] FCA 1065; (1994) 50 FCR 1.
[14] Ibid 6–7.
[15] Ibid 20–4 (Lockhart J), 31–2 (Sheppard J), 45 (Wilcox J).
[16] Ibid 2 (Lockhart and Wilcox JJ).
[17] Ibid 18–19 (Lockhart J).
[18] As had previously been held in Joos v Commissioner of Patents [1972] HCA 38; (1972) 126 CLR 611, 622–3 (Barwick CJ).
[19] [1959] HCA 67; (1959) 102 CLR 252. The joint judgment of Dixon CJ, Kitto and Windeyer JJ included obiter comments that there should be an exception for methods of medical treatment: at 270. However, this was dismissed by the majority in Anaesthetic Supplies [1994] FCA 1065; (1994) 50 FCR 1, 18–19 (Lockhart J), 44–5 (Wilcox J).
[20] Anaesthetic Supplies [1994] FCA 1065; (1994) 50 FCR 1, 18–19 (Lockhart J).
[21] Ibid 44 (Wilcox J). The three decisions of the High Court that mentioned patentability of methods of medical treatment did not directly have to deal with the question. The Court thus only made passing comments on the patentability of methods of medical treatment, and these cases do not form a strong authority in favour of exclusion. See Joos v Commissioner of Patents [1972] HCA 38; (1972) 126 CLR 611; Maeder v Busch [1938] HCA 8; (1938) 59 CLR 684; National Research Development Corporation [1959] HCA 67; (1959) 102 CLR 252.
[22] Anaesthetic Supplies [1994] FCA 1065; (1994) 50 FCR 1, 43 (Wilcox J).
[23] [2000] FCA 316; (2000) 170 ALR 439.
[24] Ibid 444 (Black CJ and Lehane J).
[25] Ibid; Anaesthetic Supplies [1994] FCA 1065; (1994) 50 FCR 1, 42 (Wilcox J).
[26] Bristol-Myers [2000] FCA 316; (2000) 170 ALR 439, 443 (Black CJ and Lehane J).
[27] Anaesthetic Supplies [1994] FCA 1065; (1994) 50 FCR 1, 18 (Lockhart J).
[28] The search was conducted on 24 May 2004, through the IP Australia website. A total of 203 513 documents were returned. See IP Australia, AU Published Patent Data Searching (2004) <http://apa.hpa.com.au:8080/ipapa/qsearch?> at 22 June 2004.
[29] Medical treatment is defined in Joos v Commissioner of Patents [1972] HCA 38; (1972) 126 CLR 611: see above n 2. I included patent applications which included a specific method of administering and treating a disease through the use of a therapeutic drug as a method of medical treatment, in accordance with Bristol-Myers (2000) 170 AR 439.
[30] Opened for signature 19 June 1978, ATS 1980 No 6 (entered into force 31 March 1980)
[31] These search results were consistent with a search I conducted under the same conditions, on 27 September 2003, where I found that 33 of the 1000 applications examined were made in relation to methods of medical treatment and that 31 of these applications were made under the PCT.
[32] Bureau of Industry Economics, The Economics of Patents, Occasional Paper 18 (1994) 34.
[33] Thomas D Mandeville, Donald M Lamberton and E J Bishop, Economic Efects of the Australian Patent System (1982).
[34] Australian Industrial Property Organisation, Industrial Property Statistics 1999–00 (2000) Table 9 <http://www.ipaustralia.gov.au/pdfs/general/ipst0099.pdf> at 27 May 2004.
[35] IP Australia, above n 28, Application numbers AU 2003279282 A1, AU 2003281245 A1, AU 2004201676 A1, AU2004201759 A1.
[36] IP Australia, above n 28, Application number AU 2004201625 A1.
[37] IP Australia, above n 28, Application number AU 2003283958 A1.
[38] IP Australia, above n 28, Application number AU 2004200594 A1.
[39] IP Australia, above n 28, Application number AU 2003278918 A1.
[40] IP Australia, above n 28, Application number AU 2004201388 A1.
[41] Australian Institute of Health and Welfare and Australasian Association of Cancer Registries, Cancer in Australia 1999, Cancer Series No 20 (2002) xiii.
[42] Ibid. Figures are for average five year relative survival proportions for all registrable cancers diagnosed in Australia for 1992 to 1997.
[43] Edward Felsenthal, ‘Medical Patents Trigger Debate Among Doctors’, The Wall Street Journal (New York), 11 August 1994, B1, B6.
[44] Brian McCormick, ‘Restricting Patents: Bipartisan Bill would Bar Ownership Claims for Medical Methods’ (1995) 38 American Medical News 3.
[45] Robert Portman, ‘Legislative Restriction on Medical and Surgical Procedure Patents Removes Impediment to Medical Progress’ (1996) 4 University of Baltimore Intellectual Property Law Journal 91, 99.
[46] Bristol-Myers [2000] FCA 316; (2000) 170 ALR 439, 480 (Finkelstein J).
[47] Ibid.
[48] Ibid.
[49] Anaesthetic Supplies [1994] FCA 1065; (1994) 50 FCR 1, 41 (Sheppard J).
[50] Loughlan, above n 10, 14.
[51] Bristol-Myers [2000] FCA 316; (2000) 170 ALR 439, 480 (Finkelstein J).
[52] Joel Garris, ‘The Case for Patenting Medical Procedures’ (1996) 22 American Journal of Law and Medicine 85.
[53] 593 CV 202 (D Vt, 1993).
[54] Portman, above n 45, 102.
[55] Seth Shulman, Owning the Future (1999) 33–4.
[56] Felsenthal, above n 43. Cf Shulman, above n 55, 33. Shulman notes that Dr Singer was informed by Dr Pallin’s legal representation that he could expect to pay between US$2500 and US$10 000 per year in royalties depending on how many times he performed the operation.
[57] Felsenthal, above n 43.
[58] Ibid.
[59] Ibid.
[60] Portman, above n 45, 102.
[61] Felsenthal, above n 43.
[62] Bristol-Myers [2000] FCA 316; (2000) 170 ALR 439, 480 (Finkelstein J). Similarly, Loughlan raises the concern that patent law may affect the value basis of medical and scientific research fields, by making members reluctant to release information or to build on prior discoveries for fear of being sued for infringement: Loughlan, above n 10, 13.
[63] Anaesthetic Supplies [1994] FCA 1065; (1994) 50 FCR 1, 40–1 (Sheppard J).
[64] Bristol-Myers [2000] FCA 316; (2000) 170 ALR 439, 480 (Finkelstein J).
[65] Garris, above n 52.
[66] Patricia Baird, ‘Patenting and Human Genes’ (1998) 41 Perspectives in Biology and Medicine 391.
[67] Bureau of Industry Economics, above n 32, 20.
[68] Edwin Mansfield, Mark Schwartz and Samuel Wagner, ‘Imitation Costs and Patients: An Empirical Study’ (1981) 91 The Economic Journal 907.
[69] Richard Levin, Richard Nelson and Sidney Winter, ‘Appropriating the Returns from Industrial Research and Development’ (1987) 3 Brookings Papers on Economic Activity 783.
[70] Mandeville et al, above n 33.
[71] Portman, above n 45, 111.
[72] Ibid. As occurred in Pallin v Singer: Shulman, above n 55, 38.
[73] The total cost of developing and approving a new drug is estimated to cost approximately US$500 million to US$800 million. See Productivity Commission, Evaluation of the Pharmaceutical Industry Investment Program, Research Report (2003)[3.4].
[74] [1994] FCA 1065; (1994) 50 FCR 1, 18.
[75] Garris, above n 52, 105.
[76] Ian Freckelton, ‘Editorial – Patenting Therapeutic Treatments and Methods’ (1994) 2 Journal of Law and Medicine 87, 90.
[77] Ibid. IP Australia concurs with this view, having stated in 1996 that if Australian patent regimes are significantly out of step with its major trading partners then it makes Australia a less desirable place for innovation and investment than overseas markets. As a consequence it was IP Australia’s view that Australian innovators would not develop the latest innovations for domestic and export markets. Similarly, the Patent Office has commented that allowing patenting across all fields encourages innovation and investment in Australia: see IP Australia, Submission to the Inquiry into Primary Producer Access to Gene Technology (1999) 3, 5.
[78] Loughlan suggests that because of the economic basis of patent law, any input from non-economists is excluded from discussion and perceived as being inadequate and of little worth: Patricia Loughlan, ‘Patents: Breaking into the Loop’ [1998] SydLawRw 24; (1998) 20 Sydney Law Review 553, 555.
[79] [1972] HCA 38; (1972) 126 CLR 611.
[80] Ibid 618.
[81] Ibid.
[82] [2002] HCA 59; [2002] 56 IPR 129.
[83] Aktiebolaget Hassle v Alphapharm [2002] HCA 59; [2002] 56 IPR 129, 155.
[85] Commissioner of Pat ents v The Wellcome Foundation Ltd (1983) 2 IPR 156, 179.
[86] Bureau of Industry Economics, above n 32.
[87] Australian Law Reform Commission, above n 5, 89–96, 189–206.
[88] Australian Institute of Health and Welfare, Australia’s Health 2002 (2002) 239 <http://www.aihw.gov.au/publications/aus/ah02/> at 22 June 2004.
[89] Commonwealth Department of Health and Aged Care, The Australian Health Care System – An Outline (2000) <http://www.health.gov.au/haf/ozhealth/ozhealth.pdf> at 22 June 2004.
[90] Ibid.
[91] Australian Institute of Health and Welfare, above n 88, 240. Public health services include the provision of acute and psychiatric hospital services, out-patient clinics, emergency departments, dental health services, infant health centres, ambulance services, community health centres and regulation of health care professionals.
[92] Commonwealth Department of Health and Aged Care, above n 89.
[93] Australian Institute of Health and Welfare , above n 88.
[94] Medicare covers people residing in Australia, who are Australian citizens, New Zealand citizens or holders of permanent visas: Commonwealth Department of Health and Aged Care, above n 89.
[95] Australian Health Care Agreement between the Commonwealth of Australia and State of New South Wales 2003–2008 (2003) Clause 46 <http://www.health.gov.au/ahca/pdf/AHCA_New_South_Wales.pdf> at 24 May 2004; Commonwealth Department of Health and Aged Care, above n 89.
[96] Commonwealth Department of Health and Aged Care, above n 89.
[97] Ibid.
[98] Ibid.
[99] Commonwealth Department of Health and Ageing, An Overview of Health Status, Health Care and Public Health in Australia, Occasional Paper Series No 5 (1999) 18.
[100] Medicare Benefits Schedule (2004) Item number 1015211 <http://www.health.gov.au/pubs/mbs/mbs6/jun04ext.txt> at 22 June 2004.
[101] Medicare Benefits Schedule, above n 100, Item number 1031518.
[102] Medicare Benefits Schedule, above n 100, Item number 1030192.
[103] Medicare Benefits Schedule, above n 100, Item number 1038504.
[104] Commonwealth Department of Health and Aged Care, above n 89.
[105] Ibid. It is also recognised that government expenditure on pharmaceutical benefits and other health services is regarded as a non-cash benefit which forms a significant part of the ‘social wage’: National Centre for Social and Economic Modelling , Deborah Schofield, Re-examining the Distribution of Health Benefits in Australia – Who Benefits from the Pharmaceutical Benefits Scheme?, Discussion Paper No 36 (1998) 1 <http://www.natsem.canberra.edu.au/pubs/dps/dp36/dp36.pdf> at 22 June 2004.
[106] Productivity Commission, above n 73.
[107] Ibid [3.2].
[108] Commonwealth Department of Health and Aged Care, above n 89.
[109] The maximum cost for general category patients is A$23.70 and for concession patients A$3.80: Department of Health and Ageing, Schedule of Pharmaceutical Benefits Efective from 1 May 2004 (2003) Explanatory notes 4 <http://www1.health.gov.au/pbs/contents/explain4.htm#what> at 22 June 2004.
[110] Productivity Commission, above n 73, [3.2].
[111] Commonwealth Department of Health and Aged Care, above n 89.
[112] Ibid. The current safety net threshold for general category patients is A$726.80 and thereafter A$3.80 per prescription for the remainder of the year. For concession patients the current threshold is A$ 197.60: Department of Health and Ageing , Schedule of Pharmaceutical Benefits Effective from 1 May 2004 (2003) Explanatory notes 5 <http://www1.health.gov.au/pbs/contents/explain5.htm#hospital> at 22 June 2004.
[113] Bristol-Myers [2000] FCA 316; (2000) 170 ALR 439, 442.
[114] Department of Health and Ageing , Schedule of Pharmaceutical Benefits Efective from 1 May 2004 (2003) Proprietary Index T <http://www1.health. gov.au/pbs/scripts/listgpindex.cfm?IndexType=P & StartCode=T> at 22 June 2004.
[115] Portman, above n 45, 118.
[116] Ann Harding et al, ‘The Distributional Impact of Government Outlays on the Australian Pharmaceutical Benefits Scheme in 2001–02’ (Paper presented at the Conference of Economists, Canberra, 29 September 2003).
[117] Ibid 8.
[118] Almost four-fifths of persons aged 55 to 64 report that they suffer from one of the national health priority conditions: Agnes Walker et al, Health and Income in Australia, Income and Wealth Report Issue No 4 (2003) <http://www.amp.com.au/au/3column/0,2338,CH5307%255FNI9922%255FSI56,00.html> at 22 June 2004.
[119] Harding et al, above n 116, 19.
[120] Generally, the existence of prescription subsidies would push up the price of pharmaceuticals because consumers are indifferent to the prices of drugs, since they pay a set price regardless of the market price, with the government covering the residual: Productivity Commission, above n 73, [3.3].
[121] K Lokuge and Richard Denniss, Trading in Our Health System? The impact of the Australia–US Free Trade Agreement on the Pharmaceutical Benefits Scheme, The Australia Institute, Discussion Paper No 55, (2003) 1.
[122] Productivity Commission, above n 73.
[123] Ibid.
[124] Ibid.
[125] Ibid.
[126] Medibank Private, What You Should Know Before Purchasing Private Health Insurance: A Key Features Guide (2004) <http://www.medibank.com.au/pdfs/key_features_guide.pdf> at 24 May 2003.
[127] Commonwealth Department of Health and Aged Care, above n 89, 266.
[128] Doctors Reform Society of Australia, Medicare Fact Sheet 2: Private Health Insurance (2001) New Doctor <http://www.drs.org.au/new_doctor/75/fact_sheet_2.html> at 22 June 2004.
[129] Commonwealth Department of Health and Aged Care, above n 89.
[130] These features are included in Medibank Private’s AdvatagePlus Hospital cover, SmartPlus Hospital cover and HealthyPlus Hospital Cover. See Medibank Private, Health Cover Products (2004) <http://www.Medibank.com.au/productandservices/healthcoverproducts.asp> at 22 June 2004. I have used Medibank Private policies in most examples regarding private health insurance because this health fund holds the highest market share at 29.7 per cent at 31 March 2004, and their policies are therefore widely held. See Private Health Insurance Ombudsman, Quarterly Bulletin No 30 (2004) <http://www. phio.org.au/publication.php?mediaid=98> at 22 June 2004.
[131] Medibank Private, above n 126.
[132] This will depend on the level of insurance and type of cover, Medibank Private, above n 126.
[133] Medibank Private, above n 126.
[134] For example Medibank Private’s Blue Ribbon Cover restricted cover will provide full cover for accommodation costs in a public hospital but only limited daily accommodation only benefits. <http://www.Medibank.com.au/productandservices/hospitalcovers/importantinfo.asp> at 22 June 2004.
[135] Deborah Schofield, National Centre for Social and Economic Modelling, Public Expenditure on Hospitals: Measuring the Distributional Impact, Discussion Paper No 37 (1998) 7.
[136] Such as intensive care, major surgery, organ transplants and specialist outpatient clinics: Commonwealth Department of Health and Aged Care, above n 89.
[137] Australian Institute of Health and Welfare, above n 88, 290.
[138] Ibid.
[139] Ibid.
[140] Ibid.
[141] Commonwealth Department of Health and Aged Care, above n 89.
[142] Ibid.
[143] Jean Bernard, Grandeur et Tentations de la Medicine (1973) 185–6.
[144] In the United States it was conceded that it is not possible to provide to everyone all health services which would be beneficial: Reinhard Priester and Arthur L Caplan, ‘Ethics, Cost Containment and the Allocation of Scarce Resources’ (1989) 24 Invest Radiol 918. A cynical view has been argued that our society has invented more beneficial health care services than we as a society can afford: see Richard Lamm, ‘Better Health Care Through Rationing’ in James M Humber and Robert F Almeder (eds), Biomedical Ethics Reviews 1994: Allocating Resources (1995) 3, 3.
[145] Lamm, above n 144, 11.
[146] Australian Institute of Health and Welfare, above n 88.
[147] Commonwealth Department of Health and Aged Care, above n 89.
[148] Schofield, above n 135.
[149] Ibid 21.
[150] Ibid 14.
[151] Ibid 21.
[152] Ibid.
[153] Ibid 24.
[154] See Jane Hall, Australian Health Policy Institute, The Public View of Private Health Insurance, Commissioned Paper Series 2001/04 (2001).
[155] Doctors Reform Society of Australia, Medicare Fact Sheet 3 – How Australia Pays for Health Care (2001) 75 New Doctor <http://www.drs.org.au/new_doctor/75/fact_sheet_3.html> at 2 June 2004.
[156] Australian Institute of Health and Welfare, Health Expenditure Australia 2001–2002, Health and Welfare Expenditure Series No 17 (2003) 6.
[157] Ibid.
[158] Australian Institute of Health and Welfare, Health Expenditure Australia 2000–2001, Health and Welfare Expenditure Series No 14 (2002) 7.
[159] Ibid.
[160] Ibid.
[161] Ibid.
[162] Bristol-Myers [2000] FCA 316; (2000) 170 ALR 439, 444 (Black CJ and Lehane J); Anaesthetic Supplies [1994] FCA 1065; (1994) 50 FCR 1, 42 (Wilcox J).
[163] Australian Institute of Health and Welfare, above n 158.
[164] Ibid.
[165] Ibid 13.
[166] Ibid.
[167] Australian Institute of Health and Welfare, above n 156, 9.
[168] However it is foreseeable that in the future, if patented methods of medical treatment become popular enough, the rise in prices caused by royalty payments may cause an increase in excess health inflation because the price of health care will presumably rise faster than prices economy wide.
[169] Kay Patterson, Minister for Health and Ageing, ‘A Fairer Medicare: Better Access, More Affordable’ (Press Release, 28 April 2003) <http://www.health.gov.au/mediarel/yr2003/kp/kp03046.htm> at 22 June 2004.
[170] This includes Pensioner Concession cardholders, Health Care cardholders and Commonwealth Seniors Health Care cardholders.
[171] Patterson, above n 169.
[172] Ibid.
[173] Ibid. Services include those provided by General Practitioners and specialists, pathology and diagnostic imaging services when performed out of hospital.
[174] Ibid.
[175] In general, changing the level of cost sharing in the community is likely to affect essential and non-essential health care. This is because risk pooling at the community level through universal insurance, such as Medicare and annual caps, protects individuals from the potentially ruinous financial consequences of health expenditure: Lokuge and Denniss, above n 121, 27.
[176] Private Health Insurance Ombudsman, above n 130.
[177] Patterson, above n 169.
[178] Private Health Insurance Ombudsman, Quarterly Bulletin No 27 (2003) <http://www.phio.org.au/publication.php?mediaid=68> at 22 June 2004.
[179] Steve Dow, ‘Safe or Sorry?’, Sydney Morning Herald (Sydney), 18 September 2003, 1.
[180] Bristol-Myers [2000] FCA 316; (2000) 170 ALR 439, 480 (Finkelstein J).
[181] For instance, of the 26.5 million people suffering from HIV and AIDS in sub-Saharan Africa, most will die without having access to pharmaceuticals. With AIDS drugs such as Combivir being sold in Zambia at prices between US$300 and US$500, clinics in the country do not have the funds to stock the drug: Terri Nderitu, ‘Balancing Pills and Patents: Intellectual Property and the HIV/AIDS Crisis’ (2001) 8(3) E Law [7] <http://www.murdoch.edu.au/elaw/issues/v8n3/nderitu83_text.html> at 22 June 2004.
[182] Australian Law Reform Commission and Australian Health Ethics Committee, Protection of Human Genetic Information, Issues Paper 26 (2001) [44].
[183] Ibid.
[184] Mark Davis and Geoff Winestock, ‘US Wants Reform of “Unfair” PBS’, The Australian Financial Review (Sydney), 13 August 2003, 5.
[185] Australian Government Department of Foreign Affairs and Trade, The Australia-United States Free Trade Agreement: Pharmaceutical Benefits Scheme <http://www.dfat.gov.au/trade/negotiations/us-fta/backgrounds/pbs.html> at 4 July 2004.
[186] Lokuge and Denniss, above n 121, 1.
[187] Productivity Commission, above n 73, [3.3].
[188] Once development costs have been recovered from the most lucrative markets, pharmaceutical companies are able to maximise profits by selling at a lower price to poorer economies, as the cost of manufacturing is minimal. Australia is classified as a nation which should be able to afford the burden of contributing to the cost of developing pharmaceuticals through higher drug prices: Alan Mitchell, ‘Drug Crisis: No Quick Fix’, The Australian Financial Review (Sydney), 7 May 2003, 62.
[189] Lokuge and Denniss, above n 121, 13.
[190] This conclusion was reached by the Prices Surveillance Authority in a report on the prices of farm chemicals in 1993: see Prices Surveillance Authority, Inquiry into the Prices of Farm Chemicals (1993).
[191] Bureau of Industry Economics, above n 32, ix.
[192] Agreement on Trade-Related Aspects of Intellectual Property Rights, opened for signature 15 April 1994, 1869 UNTS 299 (entered into force 1 January 1995).
[193] Ibid.
[194] Stephen David, ‘Real Cost of Medicines on PBS Prescription Labels’ (2003) 84 Australian Health and Medical Law News 5.
[195] However, there may be other reasons why access is denied or delayed. For instance, the unavailability of hospital beds is often considered a barrier to obtaining access to medical attention. Waiting times for elective surgery are used as an indicator of access to hospital services and in 1999–00 the median waiting time for elective surgery was 27 days. However, during the same period approximately 2.8 per cent of patients had to wait more than 12 months, with the delay generally due to a lack of beds: Australian Institute of Health and Welfare, above n 88, 293.
[196] Patents Act, 35 USC § 287 (1996); Portman, above n 45, 113.
[197] Portman, above n 45, 113.
[198] Ibid.
[199] Anaesthetic Supplies [1994] FCA 1065; (1994) 50 FCR 1, 45. Justice Wilcox stated that exclusion of methods of medical treatment from patentability involves ‘matters of ethics and social policy which the courts have no special expertise in’.
AustLII:
Copyright Policy
|
Disclaimers
|
Privacy Policy
|
Feedback
URL: http://www.austlii.edu.au/au/journals/UNSWLawJl/2004/8.html